XinKailian Biotechnology Secures U.S. Patent Authorization For Reduced Coenzyme Q10, Enabling Domestic Technology To Access Global Market
Publish Time: 2025-10-13
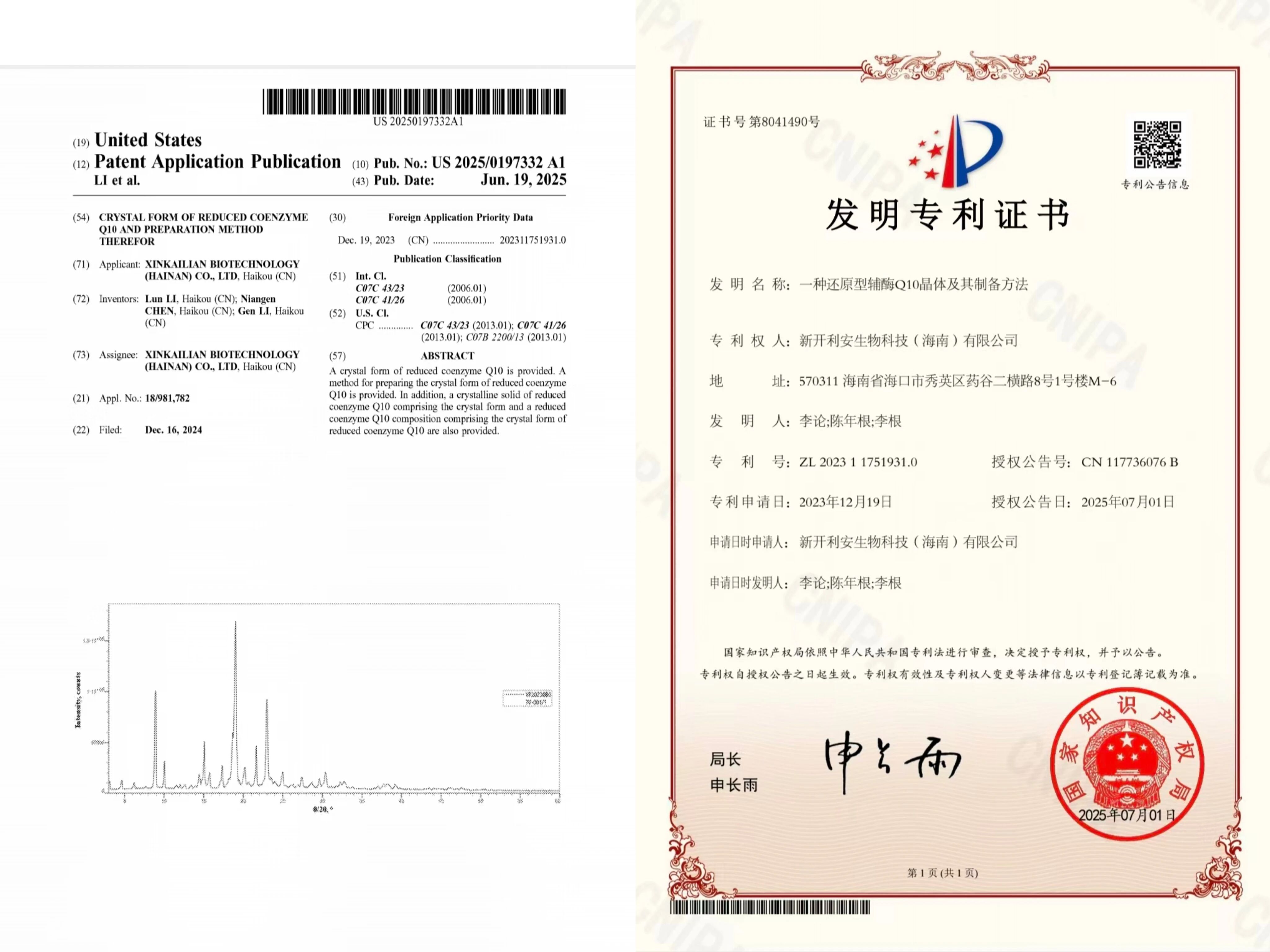
「Click the blue text to follow us」Recently, XinKailian Biotechnology (Hainan) Co., Ltd. (hereinafter referred to as "XinKailian") received an official authorization notice from the United States Patent and Trademark Office (USPTO). Its submitted patent application for "Crystal Form of Reduced Coenzyme Q10 and Its Preparation Method" (U.S. Patent No.: US20250197332A1) has passed substantive examination and successfully obtained patent authorization. This achievement marks the first time that a Chinese enterprise has broken the decades-long foreign technological monopoly in the high-end manufacturing field of coenzyme Q10, laying a crucial intellectual property foundation for domestic raw materials to enter the North American market.1. Patent Technology: A Chinese Approach to Solving Industry ChallengesThe authorized patent focuses on crystal form innovation and preparation process optimization of reduced coenzyme Q10 (ubiquinol). Its breakthroughs are mainly reflected in two major aspects:Crystal Form Characteristics,The crystal form of reduced coenzyme Q10 protected by this patent, when detected by differential scanning calorimetry, shows a characteristic endothermic peak at 52 ± 2°C, and its X-ray powder diffraction pattern also features unique crystal structure markers. This crystal form effectively addresses the issues of easy oxidation and poor stability in traditional products. Compared to oxidized coenzyme Q10, XinKailian's patented crystal form can be directly absorbed without conversion in the body, with bioavailability increased by over 70%. Moreover, when stored at room temperature, its activity loss rate is reduced by more than 60%.Preparation Process,The research and development team innovatively adopted a composite solvent system of 2-methyltetrahydrofuran and fluorinated alcohol, combined with triethylamine catalysis. This is significantly different from the traditional hexane solvent process used by Japan's Kaneka company (CN103635452B). This mild reaction pathway not only elevates the product purity to over 99.5% but also avoids the generation of harmful impurities, fully complying with the requirements of the U.S. USP43 standard and FDA registration. U.S. patent examiner Ana Muresan explicitly pointed out in the authorization opinion that this process "demonstrates non-obviousness in solvent selection and reaction control."It is worth noting that this patent claims priority based on a Chinese patent (ZL202311751931.0) submitted in December 2023, forming an "Sino-U.S. dual protection" intellectual property layout and constructing a legal barrier for the company to expand into the global market.
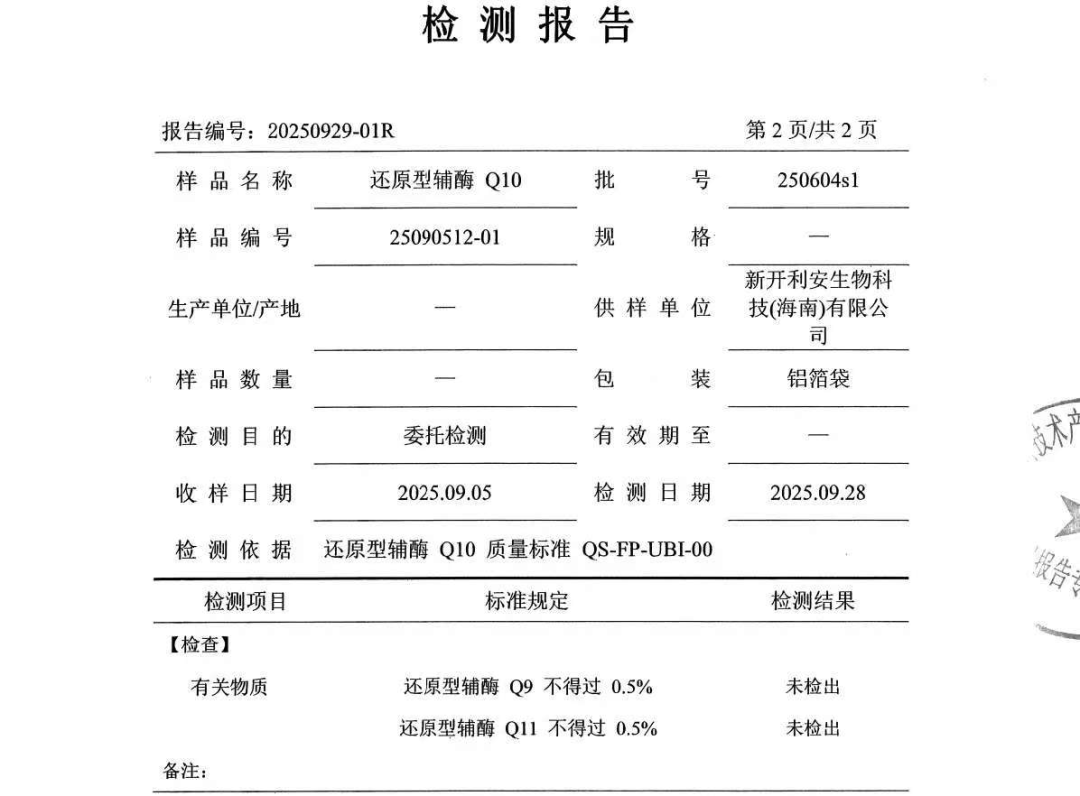
2. Industrial Implementation: From Technology Development to Mass ProductionAs the first domestic enterprise to achieve mass production of reduced coenzyme Q10, XinKailian's patent authorization is not an isolated achievement but an important part of its full-industry-chain strategy. Founded in 2022, the company has built a complete technological closed loop from "genetically engineered strains - patented crystal forms - mass production" with the support of research teams from Shanghai Jiao Tong University, Hainan Medical University, and other institutions:Strain Optimization:The genetically engineered yeast patent (ZL202410759816.6) disclosed in 2024 has increased the production of coenzyme Q10 to 160 mg/L, achieving a breakthrough in industrial application.Capacity Layout:The Yining production base, built in cooperation with Chuanning Biotechnology, has achieved an annual production capacity of 300 tons of coenzyme Q10 and plans to further expand it to 450 tons. Meanwhile, the Hainan base is planned to produce 160 tons of reduced coenzyme Q10 annually, completing full-industry-chain coverage.Quality Certification:The products have passed multiple international certifications such as ISO22000, cGMP, and Kosher, and possess an FDA registration number 16672711182, qualifying them for entry into the North American market."This patent gives us technological leverage in competition with European and American enterprises," said the person in charge of XinKailian. Currently, the company's products have entered 12 countries including Japan, Spain, Australia, New Zealand, Denmark, and Turkey through channels such as the Bangkok Asia Food Expo, the European Food Expo, the Western Natural Food & Supplement Expo in the United States, and the CPhI Worldwide in Milan, Italy. In 2024, the company's overseas revenue accounted for 40% of its total, and it is expected to exceed $100 million in 2025.3. Ultimate Purity: Defining a New Standard of QualityXinKailian's reduced coenzyme Q10 has passed tests conducted by a nationally certified third-party authoritative institution. The report confirms that the product is completely free from process-related impurities such as Q9, NK-8, and Q11. This not only verifies the advancedness and reliability of its exclusive preparation process but also ensures the high safety and excellent quality of the raw materials, providing downstream brand owners with safe and reliable top-grade raw materials.
4. Market Impact: Reshaping the Global Competitive Landscape of Coenzyme Q10For a long time, the crystal form patents and production technologies of reduced coenzyme Q10 have been monopolized by international enterprises such as Japan's Kaneka and the United States' Roche. Domestic enterprises have mostly relied on imported raw materials or engaged in low-end contract manufacturing.XinKailian's patent breakthrough is changing this situation. Its patented crystal form products not only outperform traditional products in absorption efficiency (clinical data shows that the plasma concentration can reach 4.3 μg/ml in 4 weeks) but also significantly reduce production costs, enabling domestic high-end raw materials to possess both "high quality" and "price advantages."For consumers, this technological breakthrough means improved product accessibility. Currently, XinKailian has reached raw material supply cooperation agreements with multiple international brands. It is expected that by the end of 2025, dietary supplements using its patented crystal forms will be available on mainstream health product channels in North America.With the support of the Hainan Free Trade Port policy, this enterprise, established just three years ago, is using intellectual property as a spearhead to open a new chapter in the globalization of China's biomanufacturing industry.
5. Contact InformationAddress: Building 1, M-6, No. 8 Yaogu 2nd Cross Road, Xiuying District, Haikou City, Hainan ProvinceTel: 0898-68626676Business Contacts:Mr. Qian: 18058818015Mr. Pan: 13588039509Mr. Li: 13764213222Website: https://cn.mfad.com.cn